Efficient CO2 capture by humidified polymer electrolyte membranes with tunable water state†
Abstract
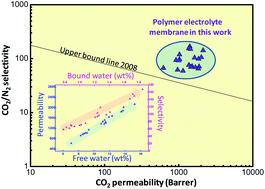
Polymer electrolyte membranes containing alkali or alkaline-earth metal salts were designed and utilized for CO2 capture. These membranes showed higher CO2 permeability than the un-doped control membrane due to the increase of water content, and CO2/gas selectivity was simultaneously enhanced due to the “salting-out” effect, which was strongly dependent on the content of bound water. More specifically, water content, water state and separation performance of polymer electrolyte membranes were strongly dependent on the salt type: (1) membranes containing alkaline-earth metal salts displayed a higher amount of bound water than those containing alkali cations, because the hydration energy of the alkaline-earth cation is relatively larger than that of the alkali cation; (2) the salts (KCl and CaCl2) that can efficiently interrupt chain packing by metal–polymer complexation facilitated the diffusion of water molecules into the polymer matrix and thus increased the total amount of absorbed water. As a consequence, CaCl2-doped membranes showed the highest CO2 permeability (2030 Barrer) and a high separation factor (108 for CO2/N2 and 31 for CO2/CH4) at 2 bar (gage pressure) and 298 K for fully humidified gas streams. The effects of annealing conditions and feed pressure were also explored to elucidate the relevant separation mechanism of the polymer electrolyte membrane.

 Please wait while we load your content...
Please wait while we load your content...