Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm†
Abstract
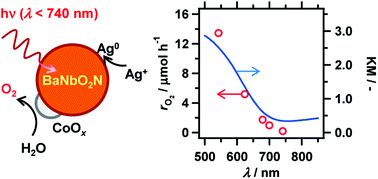
BaNbO2N was activated for photocatalytic sacrificial water oxidation and reduction by modifying the starting material for nitridation and loading appropriate cocatalysts. Addition of BaCO3 to the Ba5Nb4O15 precursor improved the crystallinity and uniformity of BaNbO2N as a nitridation product, leading to higher oxygen evolution activity. BaNbO2N generated oxygen from an aqueous AgNO3 solution under illumination up to 740 nm.

 Please wait while we load your content...
Please wait while we load your content...