Modeling an integrated photoelectrolysis system sustained by water vapor
Abstract
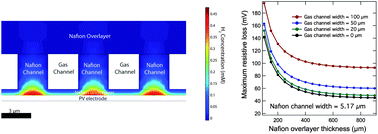
Two designs for an integrated photoelectrolysis system sustained by water vapor have been investigated using a multi-physics numerical model that accounts for charge and species conservation, electron and ion transport, and electrochemical processes. Both designs leverage the use of a proton-exchange membrane that provides conductive pathways for reactant/product transport and prevents product crossover. The resistive losses, product gas transport, and gas crossovers as a function of the geometric parameters of the two designs have been evaluated systematically. In these designs, minimization of pathways in the membrane that can support the diffusive transport of product gases from the catalyst to the gas-collecting chamber was required to prevent supersaturation of hydrogen or oxygen gases at the Nafion/catalyst interface. Due to the small, thin membrane layer that was required, a small electrode width (<300 μm) was also required to produce low resistive losses in the system. Alternatively, incorporation of a structured membrane that balances the gas transport and ionic transport allows the maximum electrode width to be increased to dimensions as large as a few millimeters. Diffusive gas transport between the cathode and anode was the dominant source for crossover of the product gases under such circumstances. The critical dimension of the electrode required to produce acceptably low rates of product crossover was also investigated through the numerical modeling and device simulations.

 Please wait while we load your content...
Please wait while we load your content...