Sodium insertion in carboxylate based materials and their application in 3.6 V full sodium cells†
Abstract
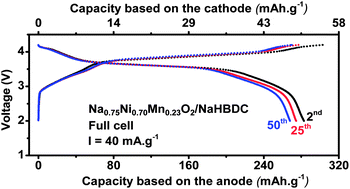
The sodium battery has the potential to be the next generation rechargeable system which utilizes cheaper and more abundant sodium material but affords nearly the same power as lithium batteries. One of the key barriers for the sodium battery is the lack of stable anode materials which can insert sodium ions reversibly at relatively low potential. This contribution reports the sodium insertion in a series of organic carboxylate based materials: (C8H4Na2O4), (C8H6O4), (C8H5NaO4), (C8Na2F4O4), (C10H2Na4O8), (C14H4O6) and (C14H4Na4O8) at low voltage (below 0.6 V vs. Na/Na+). These organic anode materials can insert reversibly up to 2 Na per molecule with good cycleability. The Na insertion mechanism was proposed and 3.6 V full sodium batteries were made and cycled reversibly at room temperature and at 55 °C.

 Please wait while we load your content...
Please wait while we load your content...