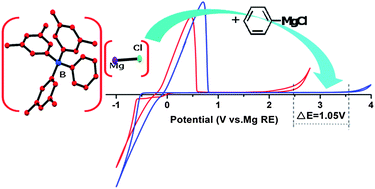
Recently, we have developed a boron based electrolyte system with outstanding electrochemical performance, formed through the reaction of tri(3,5-dimethylphenyl)borane (Mes3B) and PhMgCl in THF, for rechargeable magnesium batteries. In this paper, the main components and equilibria of the unique boron based electrolyte solutions are identified by NMR, single-crystal XRD, etc. The results prove that the solutions contain various magnesium species, such as Mg2Cl3+, MgCl+, Ph2Mg and the tetracoordinated boron anion [Mes3BPh]−. Fluorescence spectra and Raman spectroscopy analyses indicate that the high anodic stability (ca. 3.5 V vs. Mg reference electrode (RE)) of the Mes3B–(PhMgCl)2 electrolyte is attributed to the non-covalent interactions between the anion [Mes3BPh]− and Ph2Mg. Furthermore, the air sensitivity of the boron based electrolyte and its electrochemical stability on the different current collectors are studied. Finally, the reversible electrochemical process of Mg intercalation into a Mo6S8 cathode confirms that the boron based electrolyte could be practically used in rechargeable Mg battery systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...