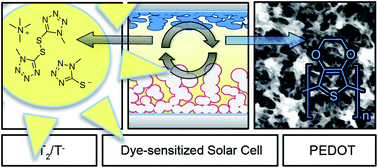
Strong scientific interests focus on the investigation of iodine-free redox couples for their application in dye-sensitized solar cells (DSCs). Recently, a disulfide/thiolate-based redox electrolyte has been proposed as a valuable alternative to the conventional I3−/I− system due to its transparent and non-corrosive nature. In the work presented herein, we systematically studied the influence of different counter electrode materials on the photovoltaic performance of DSCs employing this promising organic redox electrolyte. Our investigations focused on understanding the importance of electrocatalytic activity and surface area of the electroactive material on the counter electrode, comparing the conventional platinum to cobalt sulfide (CoS) and poly(3,4-ethylenedioxythiophene) (PEDOT). Electrochemical Impedance Spectroscopy has been used to study in detail the interfacial charge transfer reaction at the counter electrode. By using a high surface area PEDOT-based counter electrode, we finally achieved an unprecedented power conversion efficiency of 7.9% under simulated AM1.5G solar irradiation (100 mW cm−2) which, to the best of our knowledge, represents the highest efficiency that has so far been reported for an organic redox couple.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...