Structure and decomposition of zinc borohydride ammonia adduct: towards a pure hydrogen release†
Abstract
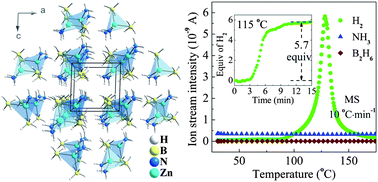
Zn(BH4)2·2NH3, a new ammine metal borohydride, has been synthesized via simply ball-milling a mixture of ZnCl2·2NH3/2LiBH4. Structure analysis shows that the subsequent complex has a monoclinic structure with unit-cell parameters of a = 6.392(4) Å, b = 8.417(6) Å, c = 6.388(4) Å and β = 92.407(4)° and space group P21, in which Zn atoms coordinate with two BH4 groups and two NH3 groups. The interatomic distances reported herein show that Zn–H bonding in Zn(BH4)2·2NH3 is shorter than Ca–H bonds in Ca(BH4)2·2NH3 and Mg–H in Mg(BH4)2·2NH3. This reduced bond contact leads to an increase in the ionic character of H. This study is able to show a good correlation between the reduced M–H distance and enhanced dehydrogenation behavior of the hydride material. Dehydrogenation results showed that Zn(BH4)2·2NH3/LiCl is able to release 5.36 wt% hydrogen (corresponding to 8.9 wt% for pure Zn(BH4)2·2NH3) below 115 °C within 15 min without concomitant release of undesirable gases such as ammonia and/or boranes, thereby demonstrating the potential of Zn(BH4)2·2NH3 to be used as a solid hydrogen storage material.

 Please wait while we load your content...
Please wait while we load your content...