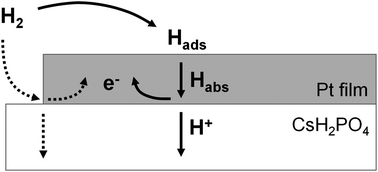
Hydrogen electro-oxidation kinetics at the Pt | CsH2PO4 interface have been evaluated. Thin films of nanocrystalline platinum 7.5–375 nm thick and 1–19 mm in diameter were sputtered atop polycrystalline discs of the proton-conducting electrolyte, CsH2PO4, by shadow-masking. The resulting Pt | CsH2PO4 | Pt symmetric cells were studied under uniform H2-H2O-Ar atmospheres at temperatures of 225–250 °C using AC impedance spectroscopy. For thick platinum films (>50 nm), electro-oxidation of hydrogen was found to be limited by diffusion of hydrogen through the film, whereas for thinner films, diffusion limitations are relaxed and interfacial effects become increasingly dominant. Extrapolation to vanishing film thickness implies an ultimate interfacial resistivity of 2.2 Ω cm2, likely reflecting a process at the Pt | H2(g) interface. Films 7.5 nm in thickness displayed a total electro-oxidation resistivity, ![[R with combining tilde]](https://www.rsc.org/images/entities/i_char_0052_0303.gif) , of 3.1 Ω cm2, approaching that of Pt-based composite anodes for solid acid fuel cells (1–2 Ω cm2). In contrast, the Pt utilization (
, of 3.1 Ω cm2, approaching that of Pt-based composite anodes for solid acid fuel cells (1–2 Ω cm2). In contrast, the Pt utilization (![[R with combining tilde]](https://www.rsc.org/images/entities/i_char_0052_0303.gif) −1/Pt loading), 19 S mg−1, significantly exceeds that of composite electrodes, indicating that the thin film approach is a promising route for achieving high performance in combination with low platinum loadings.
−1/Pt loading), 19 S mg−1, significantly exceeds that of composite electrodes, indicating that the thin film approach is a promising route for achieving high performance in combination with low platinum loadings.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[R with combining tilde]](https://www.rsc.org/images/entities/i_char_0052_0303.gif) , of 3.1 Ω cm2, approaching that of Pt-based composite anodes for solid acid
, of 3.1 Ω cm2, approaching that of Pt-based composite anodes for solid acid ![[R with combining tilde]](https://www.rsc.org/images/entities/i_char_0052_0303.gif) −1/Pt loading), 19 S mg−1, significantly exceeds that of composite electrodes, indicating that the thin film approach is a promising route for achieving high performance in combination with low platinum loadings.
−1/Pt loading), 19 S mg−1, significantly exceeds that of composite electrodes, indicating that the thin film approach is a promising route for achieving high performance in combination with low platinum loadings.

 Please wait while we load your content...
Please wait while we load your content...