Cu(OH)2
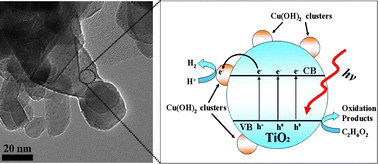
cluster-modified TiO2 (Cu(OH)2/TiO2) photocatalysts were prepared by a simple precipitation method using Degussa P25 TiO2 powder (P25) as a support and copper nitrate as a precursor. Low-power ultraviolet light emitting diodes (UV-LEDs) were used as the light source for a photocatalytic water splitting reaction. The prepared samples show especially high photocatalytic H2-production activity from aqueous solutions containing ethylene glycol as sacrificial reagent even without a Pt co-catalyst. The optimal Cu(OH)2 loading content was found to be 0.29 mol%, giving an H2-production rate of 3418 μmol h−1 g−1 with a quantum efficiency (QE) of 13.9%, which exceeded the rate on pure TiO2 by more than 205 times. This high photocatalytic H2-production activity is attributed to the presence of Cu(OH)2 clusters on the surface of the TiO2. The potential of Cu(OH)2/Cu (Cu(OH)2 + 2e− = Cu + 2OH−, Eo = −0.224 V) is slightly lower than conduction band (−0.26 V) of anatase TiO2, whilst being higher than the reduction potential of H+ (2H+ + 2e− = H2, Eo = −0.000 V), which favors the electron transfer from the CB of TiO2 to Cu(OH)2, and the reduction of H+, thus enhancing photocatalytic H2-production activity. This work not only shows a possibility for the utilization of low cost Cu(OH)2 clusters as a substitute for noble metals (such as Pt) in photocatalytic H2-production, but also for the first time exhibits a facile method for enhancing H2-production activity by using hydroxide as a co-catalyst .
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...