Cycloaddition of carbon dioxide to epoxides by highly active constrained aluminum chloride complexes†
Abstract
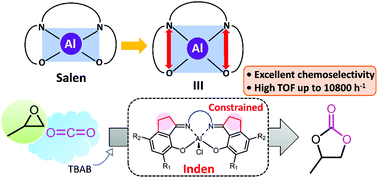
The transformation of carbon dioxide (CO2) and epoxides to cyclic carbonates has gained much interest due to its low cost, abundance, low toxicity, and renewability. Therefore, novel constrained aluminum chloride complexes were developed based on bis(salicylimine) ligands for epoxides/CO2 coupling reactions. The five-membered rings attached to the aromatic rings were designed to enlarge the coordination pocket around the aluminum center as demonstrated by single-crystal X-ray crystallography. Addition of propylene oxide (PO) to a mixture of an aluminum chloride complex and tetrabutylammonium bromide (TBAB) rapidly gave (ligand)Al–OCH(Me)CH2Cl and (ligand)Al–OCH(Me)CH2Br in similar quantities. The anion exchange between (ligand)Al–Cl and TBAB was found to be faster than the ring-opening of PO. From a series of catalyst screening and optimization, the combination of catalyst 2g having no substituent on the aromatic rings and TBAB displayed very high activity (TOF up to 10 800 h−1) for the PO/CO2 coupling reaction. This catalyst system was extended to eleven more examples of epoxides. Moreover, excellent selectivity for cyclic carbonate production was observed for both terminal and internal epoxides.


 Please wait while we load your content...
Please wait while we load your content...