Novel primary phosphinecarboxamides derived from diamines†
Abstract
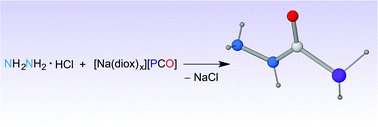
We describe the synthesis of N-functionalised phosphinecarboxamides obtained by reaction of the 2-phosphaethynolate anion (PCO−) with diamines, specifically hydrazine, methylenediamine and ethylenediamine, in the presence of acid. The resulting neutral compounds can be deprotonated to generate phosphide anions that, when further reacted with electrophiles, form secondary phosphines.


 Please wait while we load your content...
Please wait while we load your content...