Enhanced optical limiting and hydrogen evolution of graphene oxide nanohybrids covalently functionalized by covalent organic polymer based on porphyrin†
Abstract
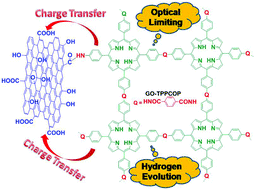
Herein, we report a novel graphene oxide (GO) nanohybrid covalently functionalized by covalent organic polymer (COP) based on porphyrin (GO-TPPCOP), as the optical limiter and hydrogen evolution reaction (HER) electrocatalyst. The GO-TPPCOP nanohybrid exhibits markedly enhanced optical limiting and HER activity over that of TPP, GO and TPPCOP alone. More importantly, the optical limiting property and HER activity of GO-TPPCOP nanohybrid are comparable to the state-of-the-art activity of materials from some previous reports. The possible mechanisms of optical limiting and HER are explored by various means, including UV-Vis absorption, fluorescence, photocurrent, electrochemical impedance spectra and Raman spectroscopic techniques. It is demonstrated that the synergistic effect and charge transfer between GO and TPPCOP are important factors in determining its optical limiting and HER performances. These results demonstrate a new strategy to design and develop functional nanohybrids for efficient optical limiting and HER activity by the covalent linkage of GO with COPs.


 Please wait while we load your content...
Please wait while we load your content...