Meta-studtite stability in aqueous solutions. Impact of HCO3−, H2O2 and ionizing radiation on dissolution and speciation†
Abstract
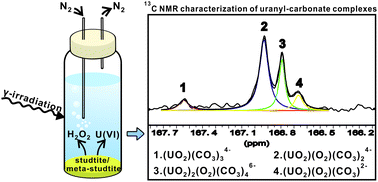
Two uranyl peroxides meta-studtite and studtite exist in nature and can form as alteration phases on the surface of spent nuclear fuel upon water intrusion in a geological repository. Meta-studtite and studtite have very low solubility and could therefore reduce the reactivity of spent nuclear fuel toward radiolytic oxidants. This would inhibit the dissolution of the fuel matrix and thereby also the spreading of radionuclides. It is therefore important to investigate the stability of meta-studtite and studtite under conditions that may influence their stability. In the present work, we have studied the dissolution kinetics of meta-studtite in aqueous solution containing 10 mM HCO3−. In addition, the influence of the added H2O2 and the impact of γ-irradiation on the dissolution kinetics of meta-studtite were studied. The results are compared to previously published data for studtite studied under the same conditions. 13C NMR experiments were performed to identify the species present in aqueous solution (i.e., carbonate containing complexes). The speciation studies are compared to calculations based on published equilibrium constants. In addition to the dissolution experiments, experiments focussing on the stability of H2O2 in aqueous solutions containing UO22+ and HCO3− were conducted. The rationale for this is that H2O2 was consumed relatively fast in some of the dissolution experiments.


 Please wait while we load your content...
Please wait while we load your content...