Ni, Pd, and Pt complexes of a tetradentate dianionic thiosemicarbazone-based O^N^N^S ligand†
Abstract
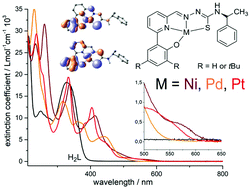
New tetradentate phenolate O^N^N^S thiosemicarbazone (TSC) ligands and their Ni(II), Pd(II) and Pt(II) complexes were studied. The diamagnetic and square planar configured orange or red complexes show reversible reductive electrochemistry and in part reversible oxidative electrochemistry at very moderate potentials. DFT calculations show essentially pyridyl-imine centred lowest unoccupied molecular orbitals (LUMO) while the highest occupied molecular orbitals (HOMO) receive contributions from the phenolate moiety, the metal d orbitals and the TSC thiolate atom in keeping with UV-vis spectroelectrochemistry. DFT calculations in conjunction with IR spectra showed details of the molecular structures, the UV-vis absorptions were modelled through TD-DFT calculation with very high accuracy. UPS is fully consistent with UV-vis absorption and TD-DFT calculated data and shows decreasing HOMO–LUMO gaps along the series Pd > Pt > Ni.


 Please wait while we load your content...
Please wait while we load your content...