Synthesis of asymmetrical diaminobis(alkoxo)-bisphenol compounds and their C1-symmetrical mono-ligated titanium(iv) complexes as highly stable highly active antitumor compounds†
Abstract
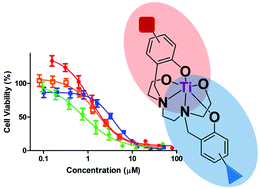
Asymmetrical 2,2′-((ethane-1,2-diylbis((2-hydroxyethyl)azanediyl))bis(methylene))diphenol substituted compounds and their C1-symmetrical diaminobis(phenolato)-bis(alkoxo) titanium(IV) complexes were synthesized, with one symmetrical analogue. X-ray crystallography corroborated tight ligand binding. Different substitutions on the two aromatic rings enabled fine-tuning of the complex properties, giving enhanced solubility, high anticancer activity (IC50 < 4 μM), and significant hydrolytic stability.


 Please wait while we load your content...
Please wait while we load your content...