Heteroleptic, polynuclear dysprosium(iii)-carbamato complexes through in situ carbon dioxide capture†
Abstract
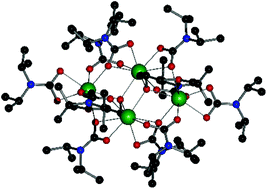
Amine groups are among the most effective systems for carbon dioxide capture. Reminiscent of the activation of nature's most abundant enzyme RuBisCO, the treatment of amines with CO2 in the presence of oxophilic metal ions, e.g. Mg2+, results in the formation of carbamates. Here we report the synthesis, structure and magnetic properties of three new dysprosium-carbamato complexes. The reaction of gaseous CO2 with N,N-diisopropylamine and DyCl3(DME)2 (DME = Dimethoxyethane) in toluene leads to the formation of the tetrametallic complex [Dy4(O2CNiPr2)10(O-C2H4–OMe)2]. The addition of 2-hydroxy-3-methoxybenzaldehyde-N-methylimine yields the hexametallic compound [Dy6(O2CNiPr2)8(O-C2H4–OMe)2(CO3)2(C9O2NH10)4] in which the metal sites form a chair-like configuration; The same hexanuclear motif is obtained using N,N-dibenzylamine. We show that by employing CO2 as a feedstock, we are able to capture up to 2.5 molecules of CO2 per Dy ion. Magnetic measurements show a decreasing χMT at low temperatures. Combining the experimental magnetic data with ab initio calculations reveils tilting of the easy axes and implies the presence of antiferromagnetic interactions between the Dy(III) metal ions.


 Please wait while we load your content...
Please wait while we load your content...