Carbazole effect on ground- and excited-state properties of rhenium(i) carbonyl complexes with extended terpy-like ligands†
Abstract
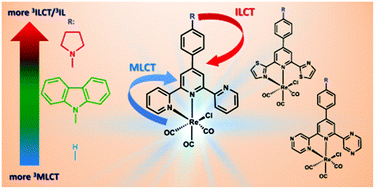
The ground- and excited-state properties of three novel complexes [ReCl(CO)3(Ln-κ2N)] bearing 2,2′:6′,2′′-terpyridine, 2,6-di(thiazol-2-yl)pyridine and 2,6-di(pyrazin-2-yl)pyridine functionalized with 9-carbazole attached to the central pyridine ring of the triimine core via phenylene linkage were investigated by spectroscopic and electrochemical methods and were simulated using density functional theory (DFT) and time-dependent DFT. To get a deeper and broader understanding of structure–property relationships, the designed Re(I) carbonyl complexes were compared with previously reported analogous systems – without any groups attached to the phenyl ring and bearing pyrrolidine instead of 9-carbazole. The results indicated that attachment of the N-carbazolyl substituent to the triimine core has less influence on the nature of the triplet excited state of [ReCl(CO)3(Ln-κ2N)] than the pyrrolidine group. Additionally, the impact of the ligand structural modifications on the light emission of the Re(I) complexes under external voltage was preliminarily examined with electroluminescence spectra of diodes containing the synthesized new molecules in an active layer.


 Please wait while we load your content...
Please wait while we load your content...