Stabilization of uranyl(v) by dipicolinic acid in aqueous medium†
Abstract
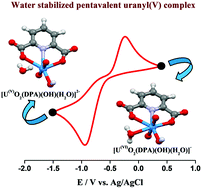
Preparation of a stable U(V) complex in an aqueous medium is a challenging task owing to its disproportionation nature (conversion into more stable U(VI) and U(IV) species) and sensitivity to atmospheric oxygen. The stable uranyl (UO22+)/dipicolinic acid (DPA) complex ([U(VI)O2(DPA)(OH)(H2O)]−) was formed at pH 10.5–12.0, which was confirmed by potentiometric and spectrophotometric titrations, and NMR, ESI-MS and EXAFS spectroscopy. The complex [U(VI)O2(DPA)(OH)(H2O)]− can be electrochemically reduced on the Pt electrode at −0.9 eV (vs. Ag/AgCl) to [U(V)O2(DPA)(OH)(H2O)]2− in aqueous medium under an anaerobic environment. According to cyclic voltammetric analysis, a pair of oxidation and reduction waves at E′0 = −0.592 V corresponds to the [U(VI)O2(DPA)(OH)(H2O)]−/[U(V)O2(DPA)(OH)(H2O)]2− redox couple and the formation of [U(V)O2(DPA)(OH)(H2O)]2− was confirmed by the electron stoichiometry (n = 0.97 ± 0.05) of the reduction reaction of [U(VI)O2(DPA)(OH)(H2O)]−. The pentavalent uranyl complex [U(V)O2(DPA)(OH)(H2O)]2− was further characterized via UV-vis-NIR absorption spectrophotometry and X-ray absorption (XANES and EXAFS) spectroscopy. The [U(V)O2(DPA)(OH)(H2O)]2− complex is stable at pH 10.5–12.0 in anaerobic water for a few days. DFT calculation shows the strong complexing ability of DPA stabilizing the unstable oxidation state U(V) in aqueous medium.


 Please wait while we load your content...
Please wait while we load your content...