Synthesis, characterization, and electrocatalytic activity of bis(pyridylimino)isoindoline Cu(ii) and Ni(ii) complexes†
Abstract
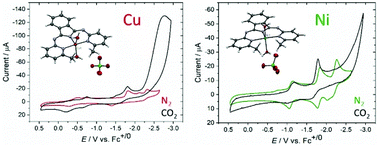
Two NNN pincer complexes of Cu(II) and Ni(II) with BPIMe− [BPIMe− = 1,3-bis((6-methylpyridin-2-yl)imino)isoindolin-2-ide] have been prepared and characterized structurally, spectroscopically, and electrochemically. The single crystal structures of the two complexes confirmed their distorted trigonal bipyramidal geometry attained by three equatorial N-atoms from the ligand and two axially positioned water molecules to give [Cu(BPIMe)(H2O)2]ClO4 and [Ni(BPIMe)(H2O)2]ClO4. Electrochemical studies of Cu(II) and Ni(II) complexes have been performed in acetonitrile to identify metal-based and ligand-based redox activity. When subjected to a saturated CO2 atmosphere, both complexes displayed catalytic activity for the reduction of CO2 with the Cu(II) complex displaying higher activity than the Ni(II) analogue. However, both complexes were shown to decompose into catalytically active heterogeneous materials on the electrode surface over extended reductive electrolysis periods. Surface analysis of these materials using energy dispersive spectroscopy as well as their physical appearance suggests the reductive deposition of copper and nickel metal on the electrode surface. Electrocatalysis and decomposition are proposed to be triggered by ligand reduction, where complex stability is believed to be tied to fluxional ligand coordination in the reduced state.


 Please wait while we load your content...
Please wait while we load your content...
