Polypyridyl Co complex-based water reduction catalysts: why replace a pyridine group with isoquinoline rather than quinoline?†
Abstract
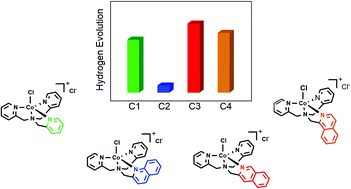
The electronic effect of the substituent has been fully leveraged to improve the activity of molecular water reduction catalysts (WRCs). However, the steric effect of the substituents has received less attention. In this work, a steric hindrance effect was observed in a quinoline-involved polypyridyl Co complex-based water reduction catalyst (WRC), which impedes the formation of Co(III)–H from Co(I), two pivotal intermediates for H2 evolution, leading to significantly impaired electrocatalytic and photocatalytic activity with respect to its parent complex, [Co(TPA)Cl]Cl (TPA = tris(2-pyridinylmethyl)-amine). In sharp contrast, two isoquinoline-involved polypyridyl Co complexes exhibited significantly improved H2 evolution efficiencies compared to [Co(TPA)Cl]Cl, benefitting mainly from the more basic and conjugated features of isoquinoline over pyridine. The dramatically different influences caused by the replacement of a pyridine group in the TPA ligand by quinoline and isoquinoline fully demonstrates the important roles of both the electronic and steric effects of a substituent. Our results may provide novel insights for designing more efficient WRCs.


 Please wait while we load your content...
Please wait while we load your content...