A broad view on the complexity involved in water oxidation catalysis based on Ru–bpn complexes†
Abstract
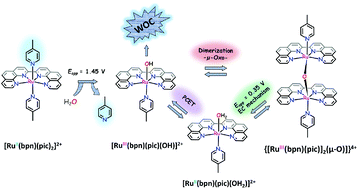
A new Ru complex with the formula [Ru(bpn)(pic)2]Cl2 (where bpn is 2,2′-bi(1,10-phenanthroline) and pic stands for 4-picoline) (1Cl2) is synthesized to investigate the true nature of active species involved in the electrochemical and chemical water oxidation mediated by a class of N4 tetradentate equatorial ligands. Comprehensive electrochemical (by using cyclic voltammetry, differential pulse voltammetry, and controlled potential electrolysis), structural (X-ray diffraction analysis), spectroscopic (UV-vis, NMR, and resonance Raman), and kinetic studies are performed. 12+ undergoes a substitution reaction when it is chemically (by using NaIO4) or electrochemically oxidized to RuIII, in which picoline is replaced by an hydroxido ligand to produce [Ru(bpn)(pic)(OH)]2+ (22+). The former complex is in equilibrium with an oxo-bridged species {[Ru(bpn)(pic)]2(μ-O)}4+ (34+) which is the major form of the complex in the RuIII oxidation state. The dimer formation is the rate determining step of the overall oxidation process (kdimer = 1.35 M−1 s−1), which is in line with the electrochemical data at pH = 7 (kdimer = 1.4 M−1 s−1). 34+ can be reduced to [Ru(bpn)(pic)(OH2)]2+ (42+), showing a sort of square mechanism. All species generated in situ at pH 7 have been thoroughly characterized by NMR, mass spectrometry, UV-Vis and electrochemical techniques. 12+ and 42+ are also characterized by single crystal X-ray diffraction analysis. Chemical oxidation of 12+ triggered by CeIV shows its capability to oxidize water to dioxygen.


 Please wait while we load your content...
Please wait while we load your content...