Zinc(ii) and copper(ii) complexes as tools to monitor/inhibit protein phosphorylation events
Abstract
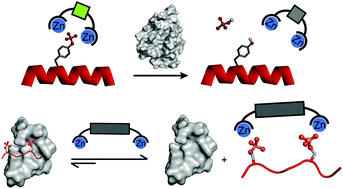
Protein phosphorylation is a key event in the signalling pathways that control most cell functions, and its deregulation is observed in many human pathologies, including inflammatory, neurodegenerative and autoimmune diseases and cancer. Compounds able to bind phosphoproteins can potentially be used as analytical tools for investigating phosphorylation-based cell signalling and/or as inhibitors of a particular signalling pathway. Metal complexes are arguably the most important class of receptors for the recognition of phosphate-containing molecules. In the last two decades the phosphate-binding ability of metal complexes has been explored for the binding and/or sensing of phosphorylated peptides and proteins. Among those we will focus this review on mono- and dinuclear copper(II) and zinc(II) complexes of varied ligand architectures used as binders of phosphorylated peptides and proteins and as sensors of phosphorylation reactions with fluorescence or other techniques in real-time. The cumulative information of strong and selective associations of the indicated receptors allowed selecting some of them for phosphoprotein/peptide enrichment and staining procedures, in vitro monitoring of kinase/phosphatase activity and disruption of phosphorylation-dependent protein–protein interactions. A perspective on the advance of this important area on the frontier between chemistry and biology is presented.
- This article is part of the themed collection: 2020 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...