Trapping of Brønsted acids with a phosphorus-centered biradicaloid – synthesis of hydrogen pseudohalide addition products†
Abstract
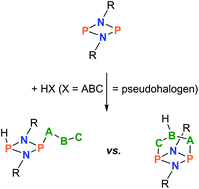
The trapping of classical hydrogen pseudohalides (HX, X = pseudohalogen = CN, N3, NCO, NCS, and PCO) utilizing a phosphorus-centered cyclic biradicaloid, [P(μ-NTer)]2, is reported. These formal Brønsted acids were generated in situ as gases and passed over the trapping reagent, the biradicaloid [P(μ-NTer)]2, leading to the formation of the addition product [HP(μ-NTer)2PX] (successful for X = CN, N3, and NCO). In addition to this direct addition reaction, a two-step procedure was also applied because we failed in isolating HPCO and HNCS addition products. This two-step process comprises the generation and isolation of the highly reactive [HP(μ-NTer)2PX]+ cation as a [B(C6F5)4]− salt, followed by salt metathesis with salts such as [cat]X (cat = PPh4, n-Bu3NMe), which also gives the desired [HP(μ-NTer)2PX] product, with the exception of the reaction with the PCO− salt. In this case, proton migration was observed, finally affording the formation of a [3.1.1]-hetero-propellane-type cage compound, an OC(H)P isomer of a HPCO adduct. All discussed species were fully characterized.


 Please wait while we load your content...
Please wait while we load your content...