Aerobic intramolecular carbon–hydrogen bond oxidation promoted by Cu(i) complexes†
Abstract
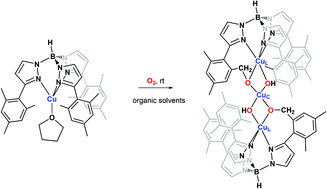
The oxidation of C–H bonds by copper centres in enzymes with molecular oxygen takes place in nature under ambient conditions. Herein we report a similar transformation in which under ambient pressure and temperature (1 atm, 25 °C) the complex TpMsCu(THF) (TpMs = hydrotris(3-mesityl-pyrazol-1-yl)borate) undergoes the intramolecular oxidation of an alkylic C–H bond with O2, leading to the formation of a trinuclear compound where alkoxy and hydroxyl ligands are bonded to the copper centres, as inferred from X-ray studies. The presence of adventitious Cu(0) derived from the partial decomposition of initial TpMsCu(THF) facilitates the formation of such a trinuclear compound. DFT studies support the reaction taking place through a Cu(III) alkoxy-hydroxyl copper intermediate.


 Please wait while we load your content...
Please wait while we load your content...