The direct and reversible hydrogenation of activated aluminium supported by piperidine†
Abstract
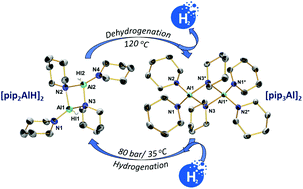
The reversible hydrogenation of aminoalanes employing activated aluminium and piperidine has been explored. A selection of transition metal (TM) compounds have been investigated as additives for producing TM-activated aluminium (TM = Ti, Zr, Hf and Y). The effect of these additives on the activation of aluminium with respect to hydrogenation of an aluminium/piperidinoalane system has been studied. It has been shown that Ti, Zr and Hf can efficiently promote the activation of aluminium for its hydrogenation. The experiments performed showed that the TM activity for the piperidinoalane formation decreases in the order Zr > Hf > Ti > Y. Using multinuclear NMR spectroscopy, the reversibility of this piperidinoalane-based hydrogenation system has been evidenced, demonstrating a potential pathway for hydrogen storage in aminoalanes. The syntheses of piperidinoalanes as well as their structural and spectroscopic characterisation are described. Single-crystal X-ray diffraction analyses of [pip2AlH]2 and [pip3Al]2 (pip = 1-piperidinyl, C5H10N) revealed dimers containing a central [AlN]2 unit.


 Please wait while we load your content...
Please wait while we load your content...