Metal organic frameworks decorated with free carboxylic acid groups: topology, metal capture and dye adsorption properties†
Abstract
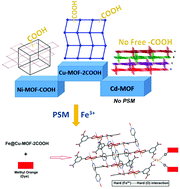
In this report, metal organic frameworks (MOFs) are designed and tuned for structural variations in order to induce metal capture which in turn directs dye adsorption properties. The three MOFs, Cu-MOF-2COOH, Ni-MOF-COOH and Cd-MOF, are synthesized by employing 1,3,5-benzenetricarboxylic acid (H3-BTC) as the main ligand and 4,4′-dipyridyl (bipy) as the spacer. The MOFs have been characterized using various spectral techniques and single crystal X-Ray studies. A topological analysis using TOPOS Pro reveals that the MOFs possess varying topologies i.e.hcb, hxl, sql and 2C1. Cu-MOF-2COOH and Ni-MOF-COOH contain two and three uncoordinated carboxylic acid groups, respectively, and in Cd-MOF, all three –COOH groups are utilized in bonding. The dye adsorption properties of the MOFs with free carboxylate group(s) were checked and we found that both MOFs are unable to adsorb any of the dyes significantly. The free carboxylate group(s) in the MOFs inspire us to elaborate their metal capturing properties. In different solvents we checked the metal capturing properties of Cu-MOF-2COOH and Ni-MOF-COOH with different metal salts. Surprisingly, both MOFs show better metal capturing properties towards the hard and highly polarizing Fe3+ ion in aqueous medium. Theoretical studies show that the free carboxylate(s) are involved in binding with metals. The post synthetically modified materials (Fe@Cu-MOF-2COOH and Fe@Ni-MOF-COOH) were further checked for their dye adsorption properties and both the doped MOFs show better adsorption properties towards the MB and MO. Furthermore, three kinetic models were employed to understand the reaction mechanism of adsorption and the pseudo second order kinetic model fits the best in both cases. The uncoordinated carboxylate groups in the channels act as post synthetic modification sites for metal capture and the post synthetically modified material thus formed attracts organic dyes following the HSAB concept. The strong interaction existing between the hard Fe3+ ion and hard donors of the dyes is responsible for the enhanced adsorption.


 Please wait while we load your content...
Please wait while we load your content...