The dominance of sulfate over two organic ligands in the solvothermal assembly of an undecanuclear cobaltous cluster: crystallography and mass spectrometry†
Abstract
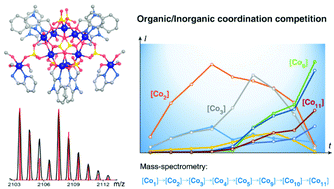
An unexpected dominance of the coordination affinity of the sulfate anion over those of two organic chelating ligands, (1-methyl-1H-benzo[d]imidazol-2-yl)methanol (Hmbm) and pyridin-2-ylmethanamine (pma), was observed during the study of the solvothermal assembly of [Co11(mbm)6(pma)2(SO4)8(H2O)6(CH3OH)6]·5CH3OH (1), from CoSO4·7H2O at 100 °C in methanol. ESI-MS of solutions at different periods of the assembly reveal a hierarchical sequence, [Co1] → [Co2] → [Co3] → [Co4] → [Co5] → [Co9], where the lower nuclearity species are richer in Co2+ and SO42− before the inclusion of more mbm ligands at the last step. Its crystal structure consists of an almost symmetrical wheel of nine cobalt atoms, [Co9(mbm)6(SO4)8(H2O)6] in edge-sharing octahedral coordination arranged in triangles with two peripheral dangling [Co(pma)(CH3OH)3]. The sulfate ions exhibit three different coordination modes, two in μ6-, two in μ3- and four as μ2-bridging. Its thermal, optical and magnetic properties are also reported.


 Please wait while we load your content...
Please wait while we load your content...