Tautomeric and conformational switching in a new versatile N-rich heterocyclic ligand†
Abstract
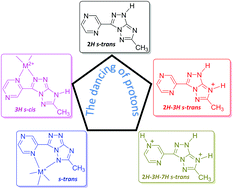
A new N-rich triazolo-triazole derivative, 4-methyl-7-(pyrazin-2-yl)-2H-[1,2,4]triazolo[3,2-c][1,2,4]triazole (C8H7N7), bearing a pyrazine residue at 7-position of the triazolo-triazole bicycle, was synthesized, and its acid–base and metal coordination properties were evaluated in solution. The results showed amphoteric behavior and the formation of stable complexes with Cu(II) and Zn(II) in pH intervals in which the ligand is neutral or deprotonated. Computational studies were performed in order to evaluate the stability of the different tautomers/conformers of the ligand, and the proton position in the neutral and acidic forms. Single crystal X-ray analysis of the free neutral ligand (2H/s-trans tautomer/conformer), and of its singly protonated (2H-3H/s-trans), doubly protonated (2H-3H-7H/s-trans) and deprotonated forms showed that the influence of the pyrazine ring on the triazolo-triazole system is mainly as electron withdrawing and chelating group, and proton acceptor. Different coordination modes have been evidenced for the neutral and deprotonated ligand. Upon metal coordination, the neutral ligand switches from 2H/s-trans to 3H/s-cis tautomer/conformer forming five-membered chelate rings, while the anionic deprotonated ligand forms six-membered chelate rings in the s-trans conformation. Altogether, five different tautomers/conformers of the ligand were isolated and characterized. In vitro tests confirmed the general antiproliferative activity of triazolo-triazole compounds and the importance of substitution in position 7 for their selectivity.


 Please wait while we load your content...
Please wait while we load your content...