Transition metal complexes of a bis(carbene) ligand featuring 1,2,4-triazolin-5-ylidene donors: structural diversity and catalytic applications†
Abstract
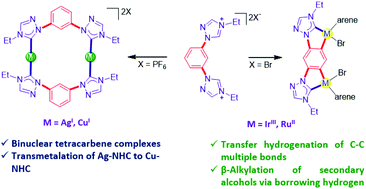
Dialkylation of the 1,3-bis(1,2,4-triazol-1-yl)benzene with ethyl bromide results in the formation of [L–H2]Br2 which, upon salt metathesis with NH4PF6, readily yields the bis(triazolium) salt [L–H2](PF6)2 with non-coordinating counterions. [L–H2](PF6)2 and Ag2O react in a 1 : 1 ratio to yield a binuclear AgI–tetracarbene complex of the composition [(L)2Ag2](PF6)2 which undergoes a facile transmetalation reaction with [Cu(SMe2)Br] to deliver the corresponding CuI–NHC complex [(L)2Cu2](PF6)2. In contrast, the [L–H2]Br2 reacts with [Ir(Cp*)Cl2]2 to generate a doubly C–H activated IrIII–NHC complex 5. Similarly, the triazolinylidene donor supported diorthometalated RuII-complex 6 is also obtained. Complexes 5 and 6 represent the first examples of a stable diorthometalated binuclear IrIII/RuII-complex supported by 1,2,4-triazolin-5-ylidene donors. The synthesized IrIII–NHC complex 5 is found to be more effective than its RuII-analogue (6) for the reduction of a range of alkenes/alkynes via the transfer hydrogenation strategy. Conversely, RuII-complex 6 is identified as an efficient catalyst (0.01 mol% loading) for the β-alkylation of a wide range of secondary alcohols using primary alcohols as alkylating partners via a borrowing hydrogen strategy.


 Please wait while we load your content...
Please wait while we load your content...