Thermodynamic analyses of the orthorhombic-to-tetragonal phase transition in Pr2−xNdxNiO4+δ under controlled oxygen partial pressures†
Abstract
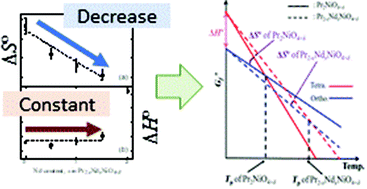
The behavior of the structural orthorhombic-tetragonal phase transition of Pr2−xNdxNiO4+δ, a candidate material for solid oxide fuel cells and oxygen permeation membranes, was investigated by differential scanning calorimetry and thermogravimetry (TG), under controlled oxygen partial pressures, P(O2). The structural phase transition temperature, TP, of Pr2−xNdxNiO4+δ increased with increasing Nd content, x, or increasing P(O2). The phase transitions of all compositions involved discrete variations in the oxygen content, Δδ, which were observed in the TG curves under various P(O2) values. Δδ of Pr2−xNdxNiO4+δ with 0.5 ≤ x ≤ 1.5 were between those of Nd2NiO4+δ and Pr2NiO4+δ, regardless of P(O2), and were slightly increased with decreasing P(O2). It was proposed that the effect of the valence change of the Pr ion on Δδ was decreased with increasing Nd content. The standard enthalpy change, ΔH°, and entropy change, ΔS°, of the phase transition were estimated from the Ellingham diagrams and van't Hoff plots, which were prepared from the relationship between P(O2) and TP using an ideal solution model. ΔS° was decreased with increasing Nd content for the specimens with 0.0 ≤ x ≤ 1.5. The ΔH° of Pr2−xNdxNiO4+δ with 0.0 ≤ x ≤ 1.5 was almost constant for all Nd contents. The increase in the phase transition temperature of Pr2−xNdxNiO4+δ with increasing x from 0.0 to 1.5 was successfully explained using the calculated values of ΔH° and ΔS°.


 Please wait while we load your content...
Please wait while we load your content...