Heterobimetallic propargyl gold complexes with π-bound copper or silver with enhanced anticancer activity†
Abstract
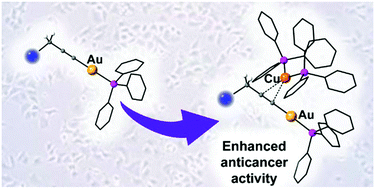
Several propargyl functionalised substrates with different heteroatoms (N, O or S) have been used for the preparation of propargyl gold(I) phosphine complexes. The complexes were prepared in high yields either by reaction of the substrate with [Au(acac)PPh3] or by reaction of [AuCl(PPh3)] with potassium hydroxide and the substrate in methanol. Several of the complexes have been characterised by X-ray diffraction showing the presence of secondary bonds such as π-stacking and aurophilic interactions. The reaction of the propargyl gold(I) phosphine complexes with [Cu(NO3)(PPh3)2] or [Ag(OTf)(PPh3)2] afforded heterobimetallic complexes with π-coordination of {Cu(PPh3)2} or {Ag(PPh3)2} to the alkyne bond. When the substituent of the propargyl unit contained more strongly coordinating pyridine moieties, [(PyCH2)2NCH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAuPPh3], coordination of the heterometal to the pyridine units occurred, displacing the phosphine groups and giving rise to a dimeric structure. The antiproliferative activity of the complexes against cisplatin resistant lung cancer cell line A549 was determined by MTT assay. The mononuclear gold complexes showed excellent activities with IC50 values < 14 μM. Coordination of copper of silver to the alkynyl fragment resulted in a significant increase in activity suggesting a synergistic effect between the two metal centres.
CAuPPh3], coordination of the heterometal to the pyridine units occurred, displacing the phosphine groups and giving rise to a dimeric structure. The antiproliferative activity of the complexes against cisplatin resistant lung cancer cell line A549 was determined by MTT assay. The mononuclear gold complexes showed excellent activities with IC50 values < 14 μM. Coordination of copper of silver to the alkynyl fragment resulted in a significant increase in activity suggesting a synergistic effect between the two metal centres.


 Please wait while we load your content...
Please wait while we load your content...