Cytotoxicity of photoactivatable bromo tricarbonyl manganese(i) compounds against human liver carcinoma cells†
Abstract
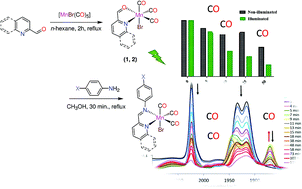
Two series of photoinduced tricarbonyl manganese(I) compounds were prepared from the reaction of [MnBr(CO)3(2-C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)] (2-C(H)
O)] (2-C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: quinoline-2-carboxaldehyde and pyridine-2-carboxaldehyde) and para-substituted aniline derivatives (X = OH, OCH3, Cl and NO2). Different electron-donating and electron-withdrawing substituents were introduced in the para-position of the phenyl ring to investigate their influence on the stability of the compounds in the dark and the photophysical properties upon illumination at 525 nm. When kept in the dark, the aerated solutions of the complexes in dimethyl sulfoxide (DMSO) and CH2Cl2 were stable. In the solution, the complexes bearing electron-withdrawing substituents, exchange their bromo ligands with DMSO solvent molecules, as evidenced from infrared and UV/Vis studies as well as time-dependent density functional theory (TDDFT) calculations. The complexes were assessed for their cytotoxicity, both in the dark and upon exposure to a 525 nm LED, against the human hepatocarcinoma cell line (HepG2). A marked reduction in the viability of HepG2 cells treated with the complex functionalized with quinoline and methoxy substituent was observed after illumination in a dose-dependent manner, with an IC50 value of 7.1 μM, making it the most phototoxic compound in our study.
O: quinoline-2-carboxaldehyde and pyridine-2-carboxaldehyde) and para-substituted aniline derivatives (X = OH, OCH3, Cl and NO2). Different electron-donating and electron-withdrawing substituents were introduced in the para-position of the phenyl ring to investigate their influence on the stability of the compounds in the dark and the photophysical properties upon illumination at 525 nm. When kept in the dark, the aerated solutions of the complexes in dimethyl sulfoxide (DMSO) and CH2Cl2 were stable. In the solution, the complexes bearing electron-withdrawing substituents, exchange their bromo ligands with DMSO solvent molecules, as evidenced from infrared and UV/Vis studies as well as time-dependent density functional theory (TDDFT) calculations. The complexes were assessed for their cytotoxicity, both in the dark and upon exposure to a 525 nm LED, against the human hepatocarcinoma cell line (HepG2). A marked reduction in the viability of HepG2 cells treated with the complex functionalized with quinoline and methoxy substituent was observed after illumination in a dose-dependent manner, with an IC50 value of 7.1 μM, making it the most phototoxic compound in our study.


 Please wait while we load your content...
Please wait while we load your content...