Highly covalent metal–ligand π bonding in chelated bis- and tris(iminoxolene) complexes of osmium and ruthenium†
Abstract
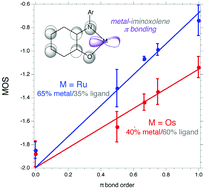
The bis(aminophenol) 2,2′-biphenylbis(3,5-di-tert-butyl-2-hydroxyphenylamine) (ClipH4) forms trans-(Clip)Os(py)2 upon aerobic reaction of the ligand with {(p-cymene)OsCl2}2 in the presence of pyridine and triethylamine. A more oxidized species, cis-β-(Clip)Os(OCH2CH2O), is formed from reaction of the ligand with the osmium(VI) complex OsO(OCH2CH2O)2, and reacts with Me3SiCl to give the chloro complex cis-β-(Clip)OsCl2. Octahedral osmium and ruthenium tris-iminoxolene complexes are formed from the chelating ligand tris(2-(3′,5′-di-tert-butyl-2′-hydroxyphenyl)amino-4-methylphenyl)amine (MeClampH6) on aerobic reaction with divalent metal precursors. The complexes’ structural and electronic features are well described using a simple bonding model that emphasizes the covalency of the π bonding between the metal and iminoxolene ligands rather than attempting to dissect the parts into discrete oxidation states. Emphasizing the continuity of bonding between disparate complexes, the structural data from a variety of Os and Ru complexes show good correlations to π bond order, and the response of the intraligand bond distances to the bond order can be analyzed to illuminate the polarity of the bonding between metal and the redox-active orbital on the iminoxolenes. The osmium compounds’ π bonding orbitals are about 40% metal-centered and 60% ligand-centered, with the ruthenium compounds’ orbitals about 65% metal-centered and 35% ligand-centered.


 Please wait while we load your content...
Please wait while we load your content...
