2,6-Diiminopyridine complexes of group 2 metals: synthesis, characterisation and redox behaviour†
Abstract
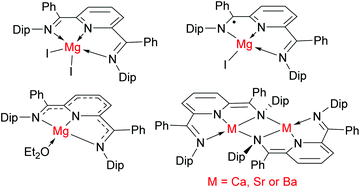
Treatment of the 2,6-diiminopyridine, NC5H3{C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(Dip)}2-2,6 (PhDimpy, Dip = 2,6-diisopropylphenyl) with [MgI2(OEt2)2] gives the adduct complex [(PhDimpy)MgI2] in which the PhDimpy ligand is neutral. This complex can be singly reduced by KC8 or a magnesium(I) complex to give [(PhDimpy˙)MgI], in which PhDimpy acts as a radical anion. Double reduction of [(PhDimpy)MgI2] in diethyl ether yields [(PhDimpy)Mg(OEt2)], in which the magnesium centre is ligated by dianionic [PhDimpy]2−. [(PhDimpy)Mg(OEt2)] can alternatively be prepared by the simple, high yielding reaction between PhDimpy and activated magnesium. A comproportionation reaction occurs between [(PhDimpy)MgI2] and [(PhDimpy)Mg(OEt2)], leading to the quantitative formation of [(PhDimpy˙)MgI]. The heavier group 2 metal dimeric complexes [{(PhDimpy)M}2] (M = Ca, Sr, Ba) can be similarly accessed by reaction of PhDimpy with the activated metal, or by KC8 reduction of in situ generated [(PhDimpy)MI2] (M = Ca, Sr). All prepared complexes have been characterised by X-ray crystallography and NMR spectroscopy. Electrochemical investigations into the complexes incorporating [PhDimpy]2− ligands reveal that they can undergo quasi-reversible 1- and 2-electron reduction processes, quasi-reversible 1-electron oxidations, and largely irreversible 2-electron oxidation events. These studies suggest that the compounds hold promise as soluble reducing agents in organic and inorganic synthesis.
N(Dip)}2-2,6 (PhDimpy, Dip = 2,6-diisopropylphenyl) with [MgI2(OEt2)2] gives the adduct complex [(PhDimpy)MgI2] in which the PhDimpy ligand is neutral. This complex can be singly reduced by KC8 or a magnesium(I) complex to give [(PhDimpy˙)MgI], in which PhDimpy acts as a radical anion. Double reduction of [(PhDimpy)MgI2] in diethyl ether yields [(PhDimpy)Mg(OEt2)], in which the magnesium centre is ligated by dianionic [PhDimpy]2−. [(PhDimpy)Mg(OEt2)] can alternatively be prepared by the simple, high yielding reaction between PhDimpy and activated magnesium. A comproportionation reaction occurs between [(PhDimpy)MgI2] and [(PhDimpy)Mg(OEt2)], leading to the quantitative formation of [(PhDimpy˙)MgI]. The heavier group 2 metal dimeric complexes [{(PhDimpy)M}2] (M = Ca, Sr, Ba) can be similarly accessed by reaction of PhDimpy with the activated metal, or by KC8 reduction of in situ generated [(PhDimpy)MI2] (M = Ca, Sr). All prepared complexes have been characterised by X-ray crystallography and NMR spectroscopy. Electrochemical investigations into the complexes incorporating [PhDimpy]2− ligands reveal that they can undergo quasi-reversible 1- and 2-electron reduction processes, quasi-reversible 1-electron oxidations, and largely irreversible 2-electron oxidation events. These studies suggest that the compounds hold promise as soluble reducing agents in organic and inorganic synthesis.


 Please wait while we load your content...
Please wait while we load your content...