Surface modification of aqueous miscible organic layered double hydroxides (AMO-LDHs)†
Abstract
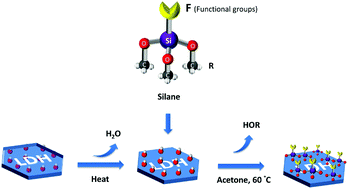
Silane modification of layered double hydroxides (LDHs) plays an important role in controlling the surface hydrophobicity and improving the compatibility of LDHs dispersed in non-polar materials. However, the surface modification of conventional LDHs in hydrous conditions typically results in aggregated particle morphologies, low surface areas and accessible pore volumes. In this study, well dispersed and high surface area silane grafted AMO-Zn2MgAl-CO3 LDH were prepared using the silane coupling agents (triethoxyvinylsilane (TEVS), triethoxyoctylsilane (TEOS) and (3-glycidyloxypropyl)trimethoxysilane (TMGPS)) in anhydrous acetone. Solution 1H NMR spectroscopy was initially used to study the rate and extent of silane reactivity with AMO-Zn2MgAl-CO3 LDH. Powder XRD, TEM, N2 BET specific surface area and total pore volume measurements showed that the structure and morphology of silane-treated AMO-Zn2MgAl-CO3 LDHs remained largely unchanged. Solid state 13C CP-MAS, 27Al DP-MAS and 29Si CP-MAS NMR spectroscopy indicates that the silanes have been successfully grafted onto the surface of the LDH. In addition to maintaining their structure, morphology, high surface area and total pore volume, these surface-functionalised LDHs are now more hydrophobic, displaying a saturation water vapour uptake (<4 wt%) that is ca. 60% lower than the untreated AMO-LDH.


 Please wait while we load your content...
Please wait while we load your content...