An iron variant of the Noyori hydrogenation catalyst for the asymmetric transfer hydrogenation of ketones†
Abstract
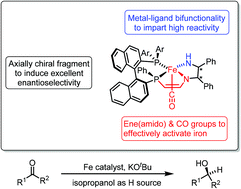
We report the design of a new iron catalyst for the asymmetric transfer hydrogenation of ketones. This type of iron catalyst combines the structural characteristics of the Noyori hydrogenation catalyst (an axially chiral 2,2′-bis(phosphino)-1,1′-binaphthyl fragment and the metal–ligand bifunctional motif) and an ene(amido) group that can activate the iron center. After activation by 8 equivalents of potassium tert-butoxide, (SA,RP,SS)-7a and (SA,RP,SS)-7b are active but nonenantioselective catalysts for the transfer hydrogenation of acetophenone and α,β-unsaturated aldehydes at room temperature in isopropanol. A maximum turnover number of 14480 was observed for (SA,RP,SS)-7a in the reduction of acetophenone. The right combination of the stereochemistry of the axially chiral 2,2′-bis(phosphino)-1,1′-binaphthyl group and the carbon-centered chiral amine-imine moiety in (SA,RP,RR)-7b′ afforded an enantioselective catalyst for the preparation of chiral alcohols with moderate to good yields and a broad functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...