Synthesis and characterization of guanidinate tin(ii) complexes for ring-opening polymerization of cyclic esters†
Abstract
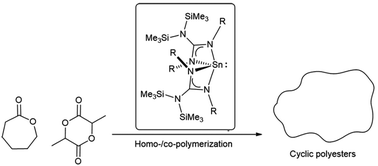
Novel homoleptic and heteroleptic (guanidinate)tin(II) complexes were successfully synthesized and structurally characterized. The first heteroleptic (guanidinato)tin(II) alkoxide complex was synthesized but found to be unstable leading to the corresponding bis(guanidinate)tin(II) complex. The catalytic activities of bis(guanidinate)tin(II) complexes having different substituents at the nitrogen atoms (isopropyl (1), cyclohexyl (2), and p-tolyl (3)) were investigated in the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) and lactide (LA). The lone-pair electrons of the tin(II) atom were proposed to act as an initiator similar to N-heterocyclic carbenes. Among the synthesized catalysts, complex 1 having less steric hindrance efficiently catalyzed both homo- and copolymerizations of ε-CL and LA giving high molecular weight cyclic polyesters. Transesterification was found to be the major contributor to the cyclization to cyclic polyesters.


 Please wait while we load your content...
Please wait while we load your content...