Water-locking molecule-assisted fabrication of nature-inspired Mg(OH)2 for highly efficient and economical uranium capture†
Abstract
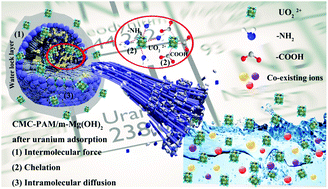
With the depletion of uranium terrestrial deposits, researchers have focused on the development of adsorbents to extract radioactive uranium from seawater/wastewater. However, the artificial manipulation of adsorbents for the cost-effective extraction of radioactive uranium from large numbers of water samples is still significantly challenging. Herein, a facile yet versatile stepwise strategy has been reported for the fabrication of adsorbents. Magnesium hydroxide (Mg(OH)2) was fabricated via the in situ conversion of a natural ore powder (magnesite), whose unique internal pore structure is highly suitable for the development of highly efficient sorbents. The coordination interaction of the synthesized adsorbent with uranium was enhanced by further introducing inexpensive molecules with water-locking properties, which resulted in superior extraction capacity and low production cost. After careful calculation, the cost per kilogram of the adsorbent was found to be about $0.21. The adsorption behaviors of the synthesized adsorbent CMC–PAM/Mg(OH)2 were investigated by batch adsorption, flow-through column adsorption (in laboratory), and field adsorption experiments in natural seawater and river. Representatively, CMC–PAM/Mg(OH)2 was exceptional in extracting uranium not only at high concentrations with sufficient capacities in a wide pH range (1584.67 mg g−1 and 454.55 mg g−1 at pH = 5 and pH = 8, respectively), but also in trace quantities including uranium in a flow-through column (55.68 mg g−1), natural seawater (8.6 mg g−1), and river (6.7 mg g−1). Inspired by this excellent performance, the effects of competitive ions on the selective adsorption of uranium by CMC–PAM/Mg(OH)2 in simulated wastewater and seawater environments were further studied. Using a combination of FTIR spectroscopic and XPS studies, it was revealed that the amine and hydroxyl groups enhanced the overall uranyl affinity of the CMC–PAM/Mg(OH)2 composite.


 Please wait while we load your content...
Please wait while we load your content...