Brønsted and Lewis acid adducts of triazenes†
Abstract
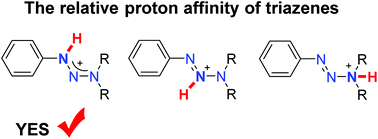
The synthetic utility of triazenes rests on the fact that the triazene function can be cleaved by Brønsted or Lewis acids, liberating diazonium compounds. However, the preferred coordination site of the acid is still a matter of debate. We have analyzed triflic acid, B(C6F5)3, and PdCl2 adducts of triazenes by NMR spectroscopy and single crystal X-ray crystallography. In all cases, we observe coordination of the acid to the N1 atom of the triazene. This finding is not only of relevance for acid-induced cleavage reactions, but also for metal-catalyzed reactions with triazenes, which are increasingly being used in synthetic organic chemistry.


 Please wait while we load your content...
Please wait while we load your content...