Effects of ligand substituents on the single-molecule magnetic behavior of quinonoid-bridged dicobalt compounds†
Abstract
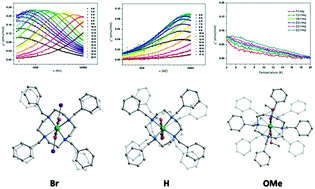
A series of quinonoid-bridged dicobalt compounds [(N4Co)2LX](ClO4)2 (1–4) (X = H, Cl, Br and OMe; N4 = 1,4,7,10-tetrabenzyl-1,4,7,10-tetraazacyclododecane) are synthesized and well characterized. Single crystal X-ray diffraction analyses reveal that the coordination geometry of one side Co in compounds 1–4 changes from a triangular prism to distorted octahedron with a change in the bridged-ligand substituent. Magnetic measurements show that compounds 1 and 3 exhibit single-molecule magnetic behavior. Magneto-structural analyses indicate that the difference in the relaxation barrier U between the four compounds results from the different orientations of the anisotropy axes of the two Co centers in the molecule.


 Please wait while we load your content...
Please wait while we load your content...