Lithium-ion storage in molybdenum phosphides with different crystal structures†
Abstract
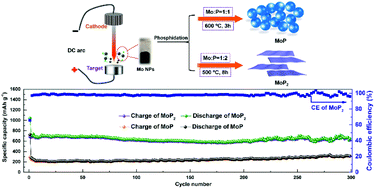
Transition metal phosphides have been receiving a great deal of attention as anode materials for Li-ion batteries due to their novel properties of high theoretical capacity and relatively low polarization. MoP and MoP2 nanoparticles with different crystal structures are synthesized by phosphorization in different stoichiometric proportions, using Mo nanospheres as the precursor produced by the plasma evaporation method. When used as the anode material for Li-ion batteries, the MoP2 electrode delivers a stable capacity of 676.60 mA h g−1 after 300 cycles at a current density of 0.1 A g−1 with obvious discharge/charge plateaus; however, the capacity of the hexagonal MoP electrode is 312.38 mA h g−1. The first-principles calculations illustrate that the di-phosphorus bond of MoP2 is prone to break and the distal P atoms preferentially bind with Li atoms to form Li3P during lithiation, but MoP prefers to form ternary LixMoP. The ex situ X-ray diffraction (XRD) and high resolution transmission electron microscopy (HRTEM) of the MoP2 electrode after cycling confirm the conversion reaction for the electrochemical storage of Li-ions.


 Please wait while we load your content...
Please wait while we load your content...