Formation of a four-bladed waterwheel-type chloro-bridged dicopper(ii) complex with dithiamacrocycle via double exo-coordination†
Abstract
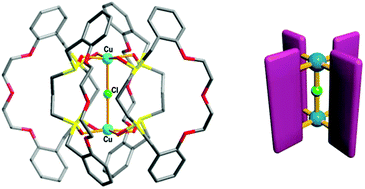
A combination of O3S2-macrocycles incorporating different sulfur-to-sulfur separations (S-(CH2)n-S, L1: n = 2, L2: n = 3) and copper(II) nitrate afforded new types of both monocopper(II) and dicopper(II) complexes, respectively. L1 gave a 1D coordination polymer [Cu2(L1)2(NO3)4]n (1) based on a convergent exo-coordination mode while L2 resulted in the formation of a divergent exo/exo-coordinated dicopper(II) complex, [Cu2(L2ox)4(μ-Cl)](NO3)4 (2), whose shape resembles a four-bladed waterwheel in which in situ oxidized macrocycles (L2ox) act as the blades and a CuII-(μ-Cl)-CuII entity corresponds to the axle shaft. The chloro-bridging ligand is derived from the dichloromethane solvent and its arrangement is held together by C–H⋯Cl− H-bonds. Compound 2 shows a weak antiferromagnetic property via the CuII-(μ-Cl)-CuII entity.


 Please wait while we load your content...
Please wait while we load your content...