Heteroleptic iron(ii) complexes with naphthoquinone-type ligands†
Abstract
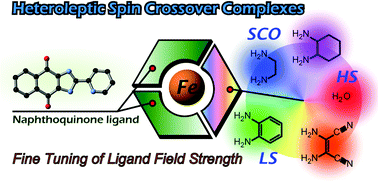
Heteroleptic iron complexes with a naphthoquinone-type ligand, 2-(pyridin-2-yl)-1H-naphtho[2,3-d]imidazole-4,9-dione (HL), were synthesized, and their structures and magnetic properties were investigated. Two spin-crossover complexes, [Fe(L)2(L1)] (1, L1 = ethylenediamine) and [Fe(L)2(L2)] (2, L2 = cyclohexanediamine) were structurally characterized at 100 K and 270 K, and their gradual spin crossover behavior observed by magnetic susceptibility measurements and confirmed by Mössbauer spectroscopic analyses. Two homologous iron(II) complexes, [Fe(L)2(L3c)] (3, L3c = diimino benzoquinone) and [Fe(L)2(L4c)] (4, L4c = iminosuccinonitrile), were found to exist in the low-spin state in the range of 10–300 K. In contrast, the corresponding complex with two water ligands, [Fe(L)2(H2O)2] (5), and the tris-chelate complex with protonated ligands (HL), [Fe(HL)3](BF4)2 (6), were found to be high-spin.


 Please wait while we load your content...
Please wait while we load your content...