A comparative study on the NOx storage and reduction performance of Pt/Ni1Mg2Al1Ox and Pt/Mn1Mg2Al1Ox catalysts
Abstract
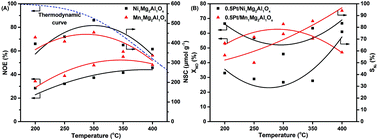
A series of Ni1Mg2Al1Ox, Mn1Mg2Al1Ox, 0.5Pt/Ni1Mg2Al1Ox and 0.5Pt/Mn1Mg2Al1Ox catalysts were carefully prepared and their NOx storage and reduction (NSR) performance including NOx oxidation efficiency (NOE), NOx storage capacity (NSC), NOx conversion rate (XNO), N2 selectivity (SN2), N2O selectivity (SN2O) and NH3 selectivity (SNH3), was systematically investigated. A SO2 resistance test was also performed in the presence of 100 ppm SO2. The NOE and NSC experimental results revealed that the Ni1Mg2Al1Ox catalyst possesses a higher NSC, while the Mn1Mg2Al1Ox catalyst possesses a better NOE. With regard to XNO, 0.5Pt/Ni1Mg2Al1Ox presented higher results at 200 °C and 400 °C, while 0.5Pt/Mn1Mg2Al1Ox obtained the highest result at 300 °C, which was more than 60% for both. In addition, compared to 0.5Pt/Ni1Mg2Al1Ox, 0.5Pt/Mn1Mg2Al1Ox exhibited a relatively higher SN2 and lower SN2O and SNH3. The NOx-TPD and H2-TPSR results indicated that NOx adsorbed on Ni1Mg2Al1Ox and 0.5Pt/Ni1Mg2Al1Ox is more stable, and that NH3 can be formed in large amounts in a lower temperature range. Both Pt-containing catalysts presented a quite stable XNO in ten cycles in the presence of 100 ppm SO2, and their SN2 can be remarkably enhanced to more than 80%, which could be attributed to the reactions of NH3-SCR and SO2 + NH3. We believe this new insight can provide a new way of thinking for the development of NSR catalysts.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...