Aerobic photolysis of methylcobalamin: structural and electronic properties of the Cbl–O–O–CH3 intermediate†
Abstract
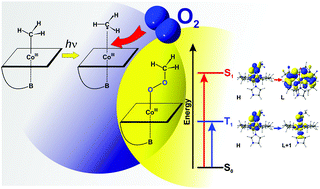
Photolysis of methylcobalamin (MeCbl) in the presence of molecular oxygen (O2) has been investigated using density functional theory (DFT) and time-dependent DFT (TD-DFT). The key step involves the formation of the Cbl–O–O–CH3 intermediate as a result of triplet O2 insertion in the Co–C bond in the presence of light. Analysis of low-lying excited states shows that the presence of light is only needed to activate the Co–C bond via the formation of the ligand field (LF) state. The insertion of O2, as well as the change in the spin state, takes place in the ground state. The analysis of the structural and electronic properties of the Cbl–O–O–CH3 intermediate is presented and possible decomposition also discussed.


 Please wait while we load your content...
Please wait while we load your content...