Gamma radiolysis of hydrophilic diglycolamide ligands in concentrated aqueous nitrate solution†
Abstract
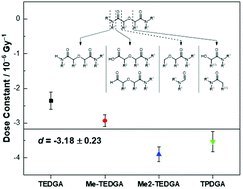
The radiation chemistry of a series of hydrophilic diglycolamides (DGAs: TEDGA, Me-TEDGA, Me2-TEDGA, and TPDGA) has been investigated under neutral pH, concentrated aqueous nitrate solution conditions. A combination of steady-state gamma and time-resolved pulsed electron irradiation experiments, supported by advanced analytical techniques and multi-scale modeling calculations, have demonstrated that: (i) the investigated hydrophilic DGAs undergo first-order decay with an average dose constant of (−3.18 ± 0.23) × 10−6 Gy−1; (ii) their degradation product distributions are similar to those under pure water conditions, except for the appearance of NOx adducts; and (iii) radiolysis is driven by hydroxyl and nitrate radical oxidation chemistry moderated by secondary degradation product scavenging reactions. Overall, the radiolysis of hydrophilic DGAs in concentrated, aqueous nitrate solutions is significantly slower and less structurally sensitive than under pure water conditions, similar to their lipophilic analogs. Acid hydrolysis, not radiolysis, is expected to limit their useful lifetime. These findings are promising for the deployment of hydrophilic DGAs as actinide aqueous phase stripping and hold-back agents, due to the presence of high concentrations of nitrate in envisioned large-scale process conditions.


 Please wait while we load your content...
Please wait while we load your content...
