Syntheses and structures of benzo-bis(1,3,2-diazaboroles) and acenaphtho-1,3,2-diazaboroles†
Abstract
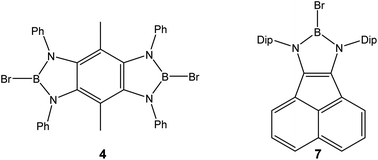
Colorless crystalline 2,6-dibromo-4,8-dimethyl-1,3,5,7-tetraphenylbenzobis(diazaborole) 4 resulted from the cyclocondensation of 3,6-dimethyl-1,2,4,5-tetraphenylaminobenzene 3d with two equivalents of boron tribromide in the presence of calcium hydride. Synthesis of the dark-red crystalline 2-bromo-N,N′-bis(diisopropylphenyl)acenaphtho-1,3,2-diazaborole 7 was effected by the cyclocondensation of 1,2-bis(N-2′,6′-diisopropylphenylimino)acenaphthene (5) and boron tribromide with subsequent sodium amalgam reduction of the initially formed burgundy red diazaborolium salt 6. Compounds 4, 6 and 7 are characterised by elemental analyses, 1H, 11B and 13C NMR spectroscopy, as well as by single X-ray diffraction studies. The electronic structures of 4, 6 and 7 are subject to DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...