Intermolecular interactions of tetrabenzoporphyrin- and phthalocyanine-based charge-transfer complexes†
Abstract
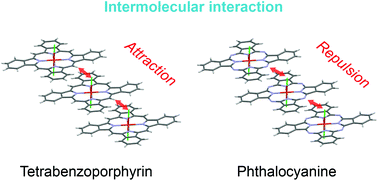
The effect of molecular modification on the intermolecular interactions in tetrabenzoporphyrin-based charge transfer complexes is reported. TPP[FeIII(tbp)Cl2]2, TPP[CoIII(tbp)Cl2]2 and TPP[CoIII(tbp)Br2]2 (TPP = tetraphenylphosphonium and tbp = tetrabenzoporphyrin) were synthesized and their crystal structures were compared to those of the reported TPP[MIII(tbp)(CN)2]2, TPP[FeIII(tbp)Br2]2 and TPP[MIII(Pc)L2]2 complexes (Pc = phthalocyanine; and L = CN, Cl or Br). The prepared CT complexes were isostructural to reported systems. However, their intermolecular interactions were found to depend on the combination of the macrocyclic (Mc) and axial ligands (L). In Pc-based systems, the overlap integral between HOMOs of Pc decreased with the increase in the size of the axial ligand, which indicated that the intermolecular interactions in Pc-based systems were dominated by repulsive interactions. On the other hand, in tbp-based systems, attractive and repulsive interactions competed with each other. Furthermore, charge transport properties were found to depend on the central metal ion as well as the combination of Mc and L, which suggested that minor molecular modifications to porphyrin complexes will cause drastic changes in both inter- and intramolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...