Mechanistic insights on the non-innocent role of electron donors: reversible photocapture of CO2 by RuII-polypyridyl complexes†
Abstract
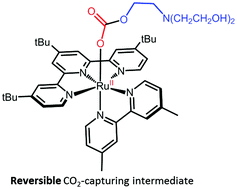
The ability of [RuII(tButpy)(dmbpy)(MeCN)]2+ (1-MeCN) to capture CO2, with the assistance of triethanolamine (TEOA), has been assessed under photocatalytically-relevant conditions. The photolability of 1-MeCN has proven essential to generate a series of intermediates which only differ by the nature of their monodentate ligand. In DMF, ligand photoexchange of 1-MeCN to give [RuII(tButpy)(dmbpy)(DMF)]2+ (1-DMF) proceeds smoothly with a quantum yield of 0.011. However, in the presence of TEOA, this process was disrupted, leading to the formation of a mixture of 1-DMF and [RuII(tButpy)(dmbpy)(TEOA)]+ (1-TEOA). An equilibrium constant of 3 was determined. Interestingly, 1-TEOA demonstrated an ability to reversibly catch and release CO2 making it a potentially crucial intermediate towards CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...