Exploring and predicting intermolecular binding preferences in crystalline Cu(ii) coordination complexes†
Abstract
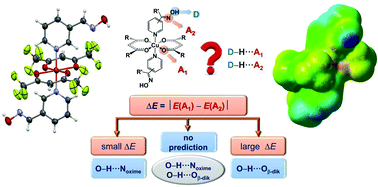
A simple model focusing on electrostatic contributions to interaction energies was found to be very effective for rationalizing the appearance of specific supramolecular interactions in a series of Cu(II) coordination compounds. The experimental space was provided by a combination of Cu(II) cations with acac-based anions (hexafluoracetylacetonato and trifluoracetylacetonato) and a series of pyridine-oxime ligands (3-pyridinealdoxime, methyl 3-pyridyl ketoxime, 4-pyridinealdoxime, methyl 4-pyridyl ketoxime, phenyl 4-pyridyl ketoxime). The calculated molecular electrostatic potential (MEP) values at competing hydrogen-bond acceptor sites, for ten structurally characterized complexes, provided guidelines for predicting supramolecular connectivity in cases when the MEP difference exceeded certain cut-off values, while two different and well-defined outcomes are possible within the so called ‘grey zone’, delineated by a range of MEP differences. It was also shown that the structural outcome within this region is determined by the influence of relatively weak, but distinct, auxillary interactions.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...